What is Electrolysis?
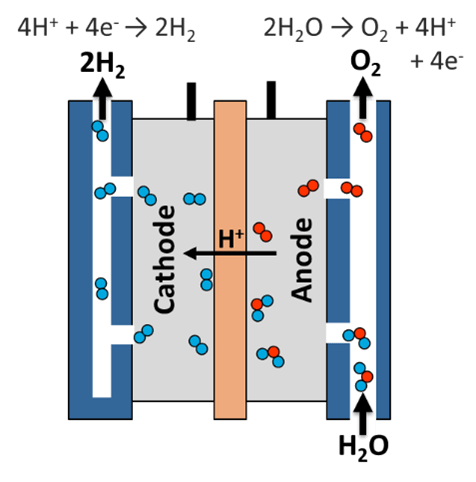
Electrolysis is the process of using electricity to break apart molecules. Electrolysis is mainly used for water and the terms electrolysis and electrolysis of water essentially mean the same thing. Water carries current on its own, but not well. To help with current flow, electrolytes are added. Electrolytes can be a variety of different molecules. For my setup I used potassium hydroxide, a strong base and thus strong electrolyte. When potassium hydroxide dissolves in water they can help current flow. When water molecules reach the anode, the oxygen molecules split off and form O2 (from 2 water molecules). This leaves 4 hydrogen and 4 electrons. The hydrogen are positively charged and go to the cathode. The hydrogen and electrons combine and form 2H2.
The Mission
The purpose of creating an electrolysis apparatus was to be able to combine my knowledge of chemistry and engineering.
Setup

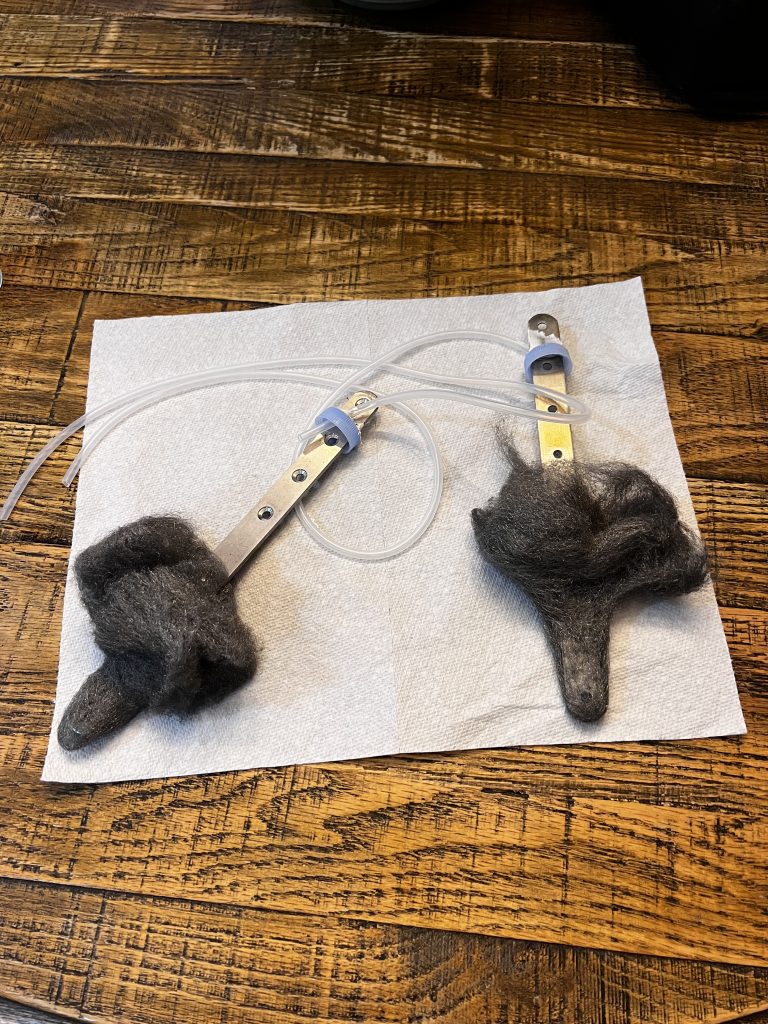






Above you can see the main components of the setup. The rods are stainless steel and steel wool was placed on them. They were then put inside bottles with the bottoms cut off. These bottles would be for collecting the gases. The lids have silicon tubing coming out of them which would be the gas outputs.



Above shows the potassium hydroxide, the bags for collecting gases, and my power supply.
Procedure
I added the whole bottle of potassium hydroxide because my container was large. This would ensure the water was very saturated with electrolytes and help with current flow. Once the potassium hydroxide flakes dissolved, it was time to turn on the power supply.
Results and Conclusion
The maximum current flow was 10 Amps at 32V. This is not very efficient and lower than I had hoped. My main issues were the distance between the anode/cathode, and the size of my container. The container was too large to saturate the water with potassium hydroxide well enough.
Recommendations
A dual output system like I have suffers from the distance between the anode and cathode. A one output system would be more efficient and compact. The problem with this is the danger of the gases being together. A single output system would create HHO, the perfectly balanced amounts of each gas for the combustion of hydrogen. While this is dangerous, a bubbler can be added to the output to make sure only the storage would blow up.
